From Johns Hopkins, Haley Johnson Reports on Alzheimer’s Research
Students at the NC State College of Veterinary Medicine have access to all kinds of internships, externships and research experiences during their four years of school. This summer, several students will be sharing some of what they're doing and learning in real time.

MONDAY, JUNE 5, 2023
For those wondering about the social things I mentioned in one of my first posts, it has all been a blast. The Chestertown tea party reenactment was so much fun and the drive out there was beautiful. It was cool to see a town on the water and drive over the Chesapeake Bay. The Wine Village at the Inner Harbor was a fun way to see the inner harbor, a portion of Baltimore that is on the water. Brew at the Zoo was a blast with live music, food vendors and local brews. I am not sure if it is just because it is summer time, but it seems like there is always something going on here, which is great!
I like to stay busy and have made plans on weekends and weeknights to see different parts of the city. There was a flea market in Patterson Park across the street from my house this past Saturday that was fun to walk around. I didn’t find anything I wanted but will have to check out the city flea market that is held every Saturday.

The other excitement of this past weekend was that my car got broken into. None of the windows were broken, and the car wasn’t damaged at all so I have no idea how they got into the car. I luckily had been advised to remove valuables from my car ahead of time so they might have gotten $3 in change I use when paying to park. Baltimore is a bigger city, and it is important to be aware of your surroundings. So far I have never felt unsafe at any point, but I also have avoided being out after dark alone.
I would recommend to anyone thinking about going to a new city for a summer experience to definitely take the opportunity to see a new place. It is important to research where you are going and make sure you are prepared and aware of what things are like ahead of time. I would highly recommend a visit to Baltimore anytime you are looking to go somewhere new! I will be going to a concert next weekend and backpacking half of the Appalachian Trail through Maryland the next weekend. Make sure you get out and live life!
WEDNESDAY, MAY 31, 2023

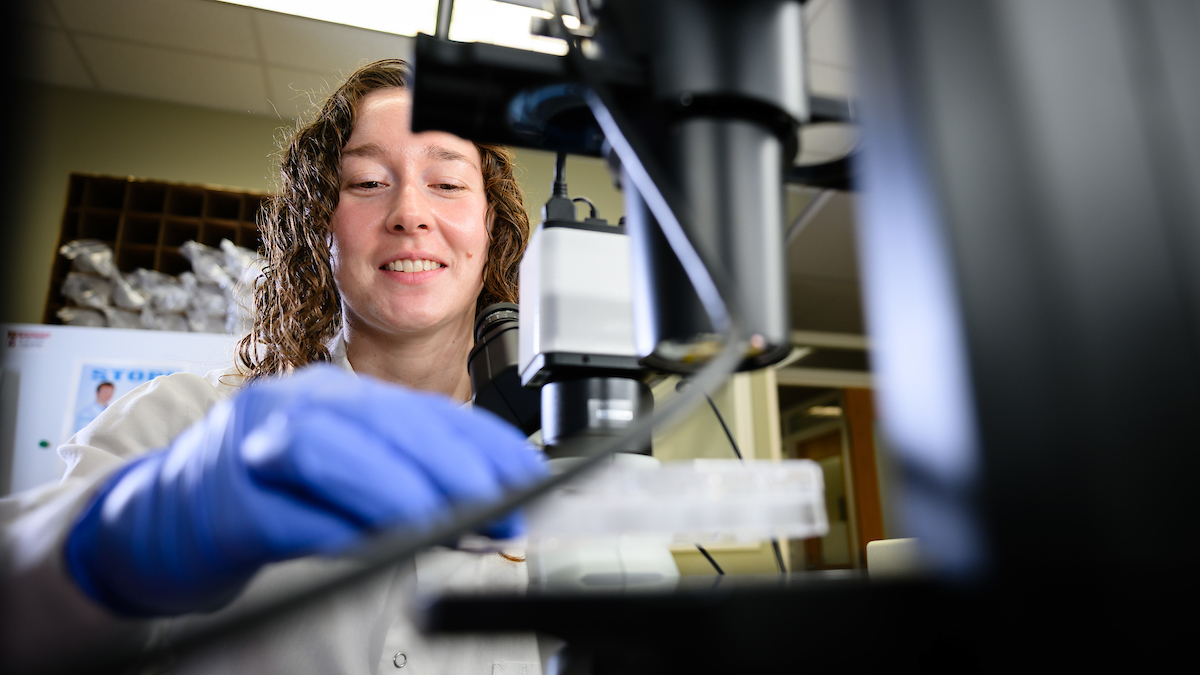
Welcome back! This week I began learning all of the different procedures I will be performing throughout the course of my experiment this summer. The main one’s include stereotaxic injections, sacrificing or euthanizing the mice, perfusing and fixing the mice brain and sectioning/mounting/viewing the hippocampus region.
The stereotaxic apparatus is a machine that holds a syringe that can inject microliter amounts of drug, dye, reagent, etc. into very specific regions of the brain. The size is about 1/20 of a raindrop so a very small amount. Right now I am practicing with GFP, or green fluorescent protein, that shows up green on the microscope after the brain is sectioned and mounted onto microscope slides.
The apparatus works off of a set of three coordinates allowing the needle to move left and right, front and back, and up and down into the brain. I am injecting into the back, bottom portion of the left hippocampus for my project. The hippocampus is located inside the temporal lobe, sort of in the middle of the brain, and is affected by a variety of neurological disorders like Alzheimer’s.
My postdoc, Mei, showed me how to do the injection in a mouse and then told me to give it a try. I was shocked and felt like there was no way I could do the injection. Another lab member was learning the injections as well and he went first, so I could see the process one more time. Then that afternoon it was my turn to do the injection. You look through a microscope as you are performing the procedure, and it took a minute to get used to seeing and using my hands while staring through the microscope.
The mouse is anesthetized in a box with equal parts oxygen and isoflurane. I then administer buprenorphine, an opioid drug used for pain management, via an intraperitoneal (IP, or within the abdomen) injection. I then shave their little head from between their eyes to the middle of their ears. Once the head is shaved, the mouse’s front teeth are situated into a mouthpiece with a hole in it. Then their head is fixed in place on both sides and their snout with a nose piece moved up providing the same anesthetic mix as was in the box. They are put on a heated plate to keep them warm during the procedure.

The shaved area is cleaned off and then a cut made with scissors in the middle of the skin on their head. This reveals the skull which is then cleaned off to remove the connective tissue between the skin and the skull. Bregma is exposed, and the stereotaxic apparatus zeroed out or calibrated at this point. Bregma is a point where two suture lines intersect toward the back of the skull. Sutures in terms of the skull are lines of connective tissue that connect the different bones of the skull together. Our skulls and animal skulls are all made up of different bony pieces that come together during development.
Once calibrated to bregma, the machine is adjusted up and to the left to the set coordinates, and the skull is marked at this point. I then have to drill a hole through the skull to expose the brain for the injection. I use a small dremel and am terrified every time I have to do this as I could easily drill into the brain. I go very slow and check how deep I have drilled many times. Once the hole is made, I draw up the green dye with the needle or nanoinjector and drop the needle down to the set depth coordinate. I slowly release the dye and let the needle sit in the brain for 10 minutes to allow the dye to be absorbed and prevent it from flowing out of the hole that the needle made going in. Once the 10 minutes are up, I remove the needle and close the skin flap using skin glue.
This process is complex, and there are many important small details to remember. It definitely has been a lot to wrap my head around, and writing out the procedure has helped some. Mei says it will get easier and I will get quicker with practice; however, knowing me the process is not going to speed up any (haha). I will go into other procedures in future posts. Hope you enjoyed all the information! 🙂
FRIDAY, MAY 26, 2023

Man! Johns Hopkins is huge and located in the sprawling city that is Baltimore. I have a 20ish minute walk to work, which is a nice way to start the day. The past few days have been orientations, completing online training modules and learning from the post-bac in the lab since the postdoc I am working under was at a conference this week.
My lab is in one of the research buildings, and our mice are housed in the basement of another research building. I practiced handling older and younger mice along with giving intraperitoneal injections or within the abdomen. This is a common injection site in mice and one must be careful to avoid puncturing an organ. A link can be found here demonstrating the injection process if you’re interested.
I have to say I was a little apprehensive about working with mice and worried about them biting me. However, this past week has been so great. The mice are all so cute, and I like getting to talk to them or give them a little tiny high-five for doing good for the injection. Is this silly? Yes. Do I care? No. I am interested in what I am doing because of the ability to work with animals and as a future veterinarian will always strive to put animal comfort and welfare at the forefront, along with having a good time.
I learned a variety of other things in the lab this past week as well, such as how to make different solutions, different staining techniques for slides and cryostat brain sectioning. Once I get into my project, we will use cryosectioning to visualize individual slices of the mouse brain, specifically looking at the hippocampus. This will allow us to see what molecules, proteins, cells or enzymes are present in which regions and if they progress through the brain.
There is a multi-day preparation process for cryosectioning that involves fixing the brain in paraformaldehyde, “washing it” or rinsing it with phosphate buffer solution (PBS) and letting it sit in a sucrose solution. Once ready it is put into a clear plastic mold filled with gel to freeze in place.
The post-bac ran me through the process a few times and then I got to try it on a brain the lab was wanting sectioned. The machine that is used is called a microtome and is kind of like a deli meat slicer you see in sub shops. However. this blade cuts extremely small slices that are 20 microns thick, think thinner than a piece of hair. We are interested in the hippocampal region of the brain that is responsible for learning and memory. You can visualize the mouse brain and hippocampus at this link here.

Once a tissue sample is cut with the microtome, it falls onto a piece of metal and has to be put on a microscope slide. You can fit 8 brain slices per individual microscope slide if you know what you are doing and can space things well. I was very happy with the six brain slices I fit per slide. We later viewed the slides and mine ended up pretty good without much damage or air bubbles present! Wooo.
This past week was the most brain dead and overstimulated I have felt in a while, and I just finished what is supposed to be the hardest year of veterinary school. There was so much new information and processes and spaces and storage places coming my way that I definitely felt like I short-circuited at times. Luckily I have time to figure everything out and process it because it definitely will take a little bit of time to understand everything.
I have plans this weekend to attend a Wine Village at the Inner Harbor, visit Chestertown for a festival and tea party reenactment, and Brew at the Zoo at the Maryland zoo in Baltimore. I am staying busy with lab work during the day and have scheduled my weekends and evenings to explore the area as much as possible.
See you next time.
MONDAY, MAY 22, 2023

Hey there, I am Haley Johnson, a rising third year at the CVM! I am interested in going into academia to conduct research and teach. After I finish my DVM I plan to pursue a Ph.D. and a residency in lab animal medicine and/or neurology. I just enjoy school so much I may never leave an academic setting, semi-joking.
I am an anatomy teaching assistant at the CVM and feel fulfilled, helpful and honestly amazing after a tutoring session or open lab where I explained different anatomical structures to students. I have been involved in research since my freshman year of undergrad at NC State and have enjoyed all the different techniques and skills I have learned since then.
Last summer I participated in the National Institutes of Health T35 Summer Veterinary Scholars research program at Wake Forest University. This summer I wanted a similar experience that was fully research-focused and allowed me to have my own project. I enjoy translational research where animal models can be used to help humans such as preclinical drug trials and want to contribute to Alzheimer’s research one day.
When figuring out where I wanted to go this summer, Johns Hopkins wasn’t a school that popped to mind immediately. I actually found out about the opportunity through an email sent by staff at the CVM informing students about it. I knew I wanted to go to a different state this summer and, after realizing Johns Hopkins has a T35 program, I was thrilled to apply.
I was even more thrilled when I found out the different project options for the summer program. The lab where I ended up getting placed works in drug discovery and is looking at experimental Alzheimer’s drugs! I am looking forward to getting started and meeting everyone. The lab is quite big so there will be lots of opportunities to learn different experimental techniques and diagnostic assays.
I will be working with mice this summer. I have no prior experience with mice and find it somewhat daunting as I will be injecting things into a very specific region of their brains. However, one of my mentors at the CVM recommended that I reach out to NC State’s lab animal department to see if I could get some mouse-handling experience before I left for the summer. The head lab animal veterinarian and resident were fantastic and set up time for me to learn basic handling and injection techniques the next day. It really helped me to feel more confident going into my summer experience and helped me feel a bit more at ease.
Today I packed up my car with my bike and kayak and hit the road for Baltimore, Maryland. I found a room through Johns Hopkins subleasing facebook page in an old Polish convent near Patterson Park. Unfortunately, street parking is competitive here and I have had to become a master at parallel parking (closest parallel park so far pictured below). The area I am staying in is called Butcher’s Hill, and I enjoyed walking around the area a bit on my first day here. I did get a parking ticket in less than 24 hours but hopefully won’t get another one this summer! I am looking forward to starting in the lab tomorrow and getting to see Johns Hopkins.
Until next time.

- Categories: